Why is CAR T cell therapy seen as the future of cancer treatment?
By Dr Sourabh Radhakrishnan, Senior Consultant and Director, Medical and Precision Oncology, Karkinos Healthcare
CAR T cell therapy, also known as Chimeric Antigen Receptor T cell therapy, is considered as a groundbreaking treatment that harnesses the power of a patient’s own immune system to fight certain types of cancer.
These therapies are primarily used for treating blood cancers, including lymphomas, certain forms of leukaemia, and, most recently, multiple myeloma. Clinical trials conducted globally have shown promising results in end stage patients, especially in patients suffering from Acute Lymphocytic Leukaemia.
T cells and the discovery of CAR T cell therapy
Before we delve into the details of CAR T cell therapy, it is essential to know what T cells are and how they are engineered to be used in immunotherapy.
T cells are a type of white blood cells that play a central role in the immune system. They are responsible for identifying and destroying infected cells and cancer cells. T cells develop in the thymus, a gland located in the chest. Once mature, they circulate in the bloodstream and lymphatic system, searching for foreign invaders.
T cells play a pivotal role in orchestrating immune responses and directly eliminating cells infected by pathogens. These cells recognise foreign invaders (such as viruses, bacteria, and cancer cells) and mount an immune attack against them.
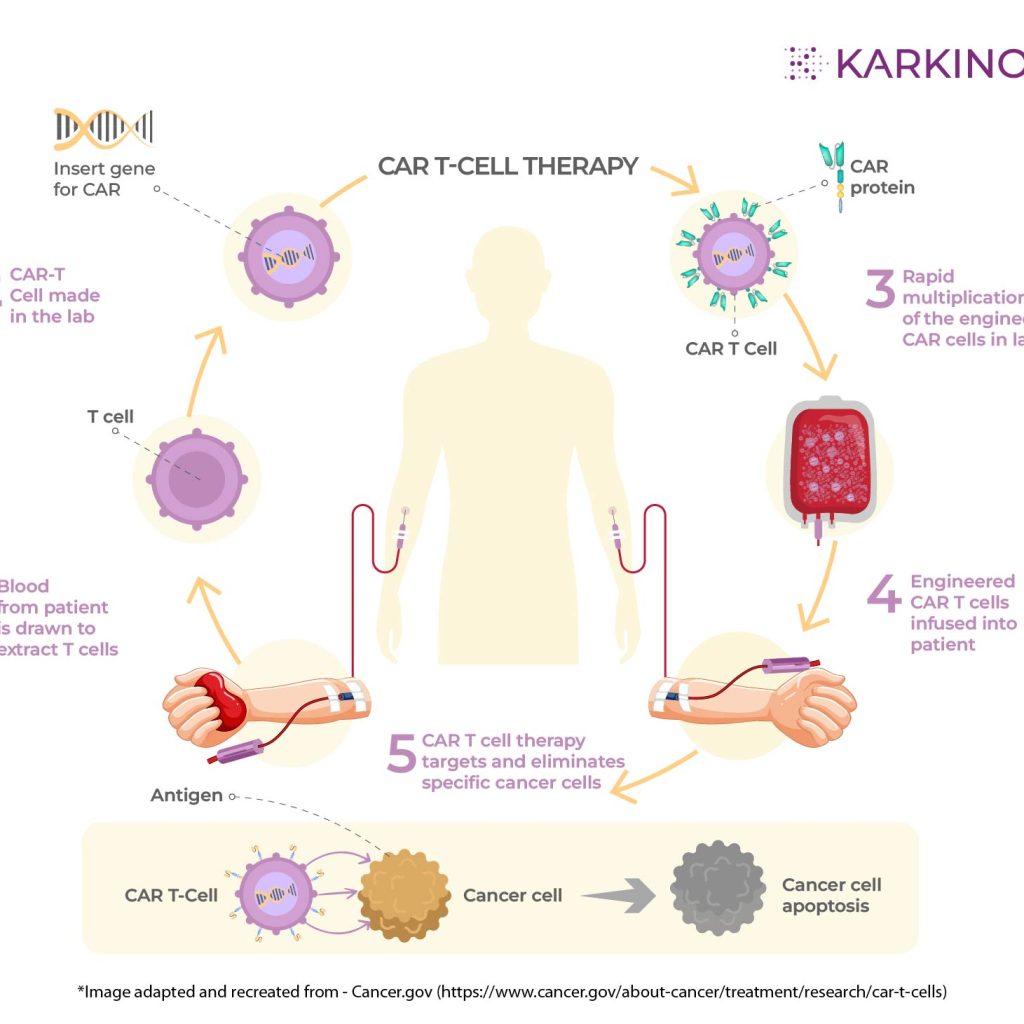
CAR T cell therapy is a type of immunotherapy that uses genetically engineered T cells to fight cancer. In the laboratory, the collected T cells from a cancer patient are genetically modified to produce special receptors called Chimeric Antigen Receptors (CARs).
In the laboratory, the T cells extracted from the patient’s blood is changed by adding a lab-made gene for a chimeric antigen receptor. Next, they make the new CAR T cells grow and multiply until there are enough cells to target cancerous cells effectively.
The CARs are designed in such a way to recognize specific proteins (antigens) found on the surface of cancer cells. After genetic modification, the CAR T cells are infused back into the patient’s bloodstream.
The modified CAR T cells now have receptors that allow them to bind specifically to cancer cells. Once attached to cancer cells, the CAR T cells activate the immune response. They release signals that attract other immune cells and initiate a powerful attack against the cancer.
These modified T cells are then able to multiply and kill cancer cells throughout the body. A CAR is a molecule that combines an antibody fragment that can recognize a specific antigen on cancer cells with a T cell signalling domain that activates the T cell.
The first clinical trial of CAR T cell therapy was conducted in the early 2000s. These trials showed that CAR T cell therapy was safe and could produce some positive results in patients with cancer. However, it was not until the late 2010s that CAR T cell therapy became a widely used treatment for cancer.
Why is CAR T cell therapy known as a ‘living drug’?
CAR T-cell therapy gets the nickname ‘living drug’ for a few reasons. As it is made from living cells, unlike traditional medications that are chemicals or compounds, CAR T-cell therapy uses a patient’s own immune cells, specifically T cells. Because they’re living cells, they can grow, multiply, and continue fighting cancer cells for an extended period.
Also, it is recognized as personalized medicine as the therapy is customized for each patient. Engineering the T cells to recognise and attack a specific marker on the patient’s specific type of cancer cells gives it the personalised approach. This personalisation increases the effectiveness of the treatment.
How does CAR T cell therapy work in the fight against cancer?
The main function of T cells, the white blood cells in your immune system, is to monitor and defend the body from any form of foreign agents (abnormal cells, bacteria, virus, fungi, etc.), by tracking proteins called antigens on the surface of intruder cells.
The T cells have their own surface proteins called receptors. These receptors can recognize cells that have abnormal antigens. T cells act as a surveillance system for abnormal cells, becoming active when a receptor recognizes an abnormal cell. The activated T-cell goes to work, destroying the abnormal cell and activating other parts of your immune system to help find and destroy abnormal cells.
But the T-cell receptors don’t always detect cancerous cells. This is where CAR T cells, engineered in the laboratory, are made to recognize a specific antigen on the surface of cancer cells. Once the CAR T cells are injected into the human bloodstream, the CAR T cell receptors detect and destroy cancerous cells.
The cells also keep on multiplying to have a long-term supply of CAR T cells targeting specific cancer cells. That long-term supply is why researchers and healthcare providers call CAR T-cell therapy a type of living drug.
Significant timelines of CAR T cell therapy
The discovery of CAR T cell therapy is generally credited to Memorial Sloan Kettering Cancer Center (MSK) researchers Zelig Eshhar, Michel Sadelain, and Steven Rosenberg in the late 1980s. These researchers developed a way to genetically modify T cells to express a chimeric antigen receptor (CAR).
1992
As a postdoctoral student at the Whitehead Institute at MIT, immunologist Michel Sadelain begins using newly developed genetic engineering tools, specifically retroviral vectors, to introduce genes into T cells, with the goal of making cancer fighting cells.
1994
The discovery takes a significant step forward when MSK scientists learn to isolate virus-specific T cells for use in stem cell transplants. These cells help prevent post-transplant infections and virally caused cancers in patients, and also limit graft-versus-host disease, a dangerous side effect.
2002
The MSK team builds the first effective CAR T cells, targeted against a prostate cancer antigen. These second-generation CARs T cells are able to survive, proliferate, and kill prostate cancer cells in the lab, establishing the feasibility of CAR T cell therapy. An even more promising CAR target lay ahead.
2012
The Cancer Research Institute bestows the 2012 Coley Award for Tumour Immunology, jointly, to Michel Sadelain of MSK and Carl June of the University of Pennsylvania for their work contributing to the development of CAR T therapy.
2013
MSK physician-scientist Renier Brentjens and colleagues publish results of a clinical trial using CD19 CAR T cells in adults with acute lymphoblastic leukemia (ALL). This is the first published study using CARs to treat ALL in humans.
2013
Science magazine votes cancer immunotherapy the 2013 “Breakthrough of the Year.” Among the approaches discussed is CAR T cell therapy.
2014
The FDA grants Breakthrough Designation status to CD19-directed CAR T cells, signalling the field’s scientific and clinical progress.
2014
MSK physician-scientist Prasad Adusumilli publishes results on CARs built to recognize an antigen on solid tumours called mesothelin. Solid tumours require a different approach since they do not make CD19.
2017
Dr. Sadelain and colleagues show that the genome-editing tool CRISPR can be used to place a CAR at a specific genetic location in T cells, for improved functioning.
2017
The FDA approves CD19-directed CAR T cells for the treatment of relapsed, refractory acute lymphoblastic leukaemia in children and young adults.
Source: https://www.mskcc.org/timeline/car-t-timeline-progress
Advantages and disadvantages of CAR T cell therapy
CAR T-cell therapy holds immense promise for the future of cancer treatment owing to a few advantages:
- High remission rates: CAR T cells have shown success in treating certain cancers, particularly blood cancers like leukaemia and lymphoma. Studies have shown remission rates as high as 80% in some cases, exceeding what traditional therapies can achieve.
- Personalised approach: Unlike chemotherapy or radiation that target all rapidly dividing cells, CAR T cells are programmed to go after a specific antigen on cancer cells. This personalisation minimises harm to healthy tissues and reduces side effects.
- Living drug: As mentioned earlier, CAR T cells are engineered to multiply and persist within the body. This means they can offer long-term protection against cancer recurrence. They act like a living defence system continuously patrolling for and eliminating cancer cells.
However, there are also challenges to address:
- Manufacturing complexity: Creating CAR T cells is a complex process that can be expensive and time-consuming. Researchers are working on streamlining the manufacturing process to make it more accessible.
- Solid tumour application: CAR T cell therapy has shown great results in blood cancers like leukaemia and lymphoma. However, solid tumours pose a different challenge. Solid tumours often lack ideal targets (antigens) for CAR T cells, and the tumour microenvironment can suppress the activity of these engineered cells.
- Side effects: CAR T-cell therapy can cause severe side effects like cytokine release syndrome (CRS) where the immune system goes into overdrive. Managing these side effects is crucial for patient safety. Another concern is “off-target” effects, where CAR T cells attack healthy tissue due to targeting the wrong antigen. Research is ongoing to improve the safety profile of this therapy.
- Durability and Persistence: While some patients experience long-term remission, CAR T cells can sometimes become inactive or ineffective over time. This can lead to relapse. Researchers are looking at ways to enhance the persistence and memory function of these cells.
- Overcoming Tumour Escape: Cancer cells can mutate and lose the targeted antigen, rendering CAR T cells useless. Developing strategies to target multiple antigens or using smart CAR T cells that can adapt to these changes are areas of active research.
Not cost-effective: The process of developing this personalised therapy makes the cost of treatment very high. It is beyond reach for the common man to avail this treatment.
Hence, CAR T cell therapy needs further research to improve its effectiveness against solid tumours, minimise side effects, enhance durability, and make it more affordable and accessible. Overcoming challenges related to tumour escape mechanisms is also crucial for long-term success.
India’s progress in CAR T cell therapy
India has worked on and has made significant strides in CAR-T cell therapy in recent years, emerging as a frontrunner among developing countries. In June 2021, Tata Memorial Hospital (TMH) released the news that it had conducted the first CAR T cell therapy at the Bone Marrow Transplant unit at ACTREC in Mumbai. The CAR T cells were designed and manufactured at the Bioscience and Bioengineering (BSBE) department of IIT Bombay.
This initiative was partly supported by the BIRAC-PACE scheme. The TMC-IIT Bombay team together are further supported to extend this project for conducting Phase I/II trials of their CAR-T product by DBT/BIRAC, through the National Biopharma Mission.
This is the ‘first-in-India’ gene therapy and the central government’s National Biopharma Mission-BIRAC had approved Rs 19.15 crore to the team for conducting a first-in-human phase-1/2 clinical trial of the CAR-T cells.
Another major breakthrough came in October 2023 with the approval of NexCAR19, the first indigenously developed CAR-T cell therapy by ImmunoACT (a company incubated at IIT Bombay) in collaboration with Tata Memorial Hospital. This therapy targets B-cell cancers like leukaemia and lymphoma, offering a promising and potentially more affordable option for patients within India’s healthcare system.
Much recently, on April 4th 2024, the President of India, Smt Droupadi Murmu launched India’s first homegrown gene therapy for cancer at Indian Institute of Technology, Bombay. Speaking on the occasion, the President said that the launch of India’s first gene therapy is a major breakthrough in our battle against cancer. She expressed confidence that it will be successful in giving new lives to countless patients.
The President said that CAR-T cell therapy is considered to be one of the most phenomenal advances in medical science. It has been available in the developed nations for some time, but it is extremely costly, and beyond the reach of most patients around the world. She was happy to note that the therapy being launched on April 4th is the world’s most affordable CAR-T cell therapy.
The President was happy to note that India’s first CAR-T cell therapy is developed through collaboration between the Indian Institute of Technology, Bombay and Tata Memorial Hospital WCI in association with industry partner ImmunoACT. She said that this is a praiseworthy example of academia-industry partnership, which should inspire many more similar efforts.
Also, India is actively conducting clinical trials to assess the efficacy and safety of various CAR-T cell therapies. For instance, trials are ongoing for Actalycabtagene autoleucel (Actaly-cel) for adult Acute Lymphoblastic Leukaemia (ALL), Varnimcabtagene autoleucel (var-cel) for relapsed/refractory B-cell malignancies, and PRG1801 for multiple myeloma.
The National Biopharma Mission is also supporting the development of Lentiviral vector manufacturing facility for packaging plasmids used to transfer the modified T cell inside the body, cGMP facility for T-cell transduction and expansion for CAR T-cell manufacturing to two other organizations. The development of CAR-T cell technology for diseases including acute lymphocytic leukaemia, multiple myeloma, glioblastoma, hepatocellular carcinoma, and type-2 diabetes is supported through DBT.
Work in progress
Undoubtedly, with CAR T cell therapy an exciting new era in cancer treatment awaits. Researchers around the world are constantly working on novel ways to develop new and improved CAR T cell therapies. Optimizing CAR T cell design and delivery could lead to cures for more malignancies. They are also working to make CAR T cell therapy more affordable and accessible to more patients.
Researchers are also exploring a way to work around allogeneic CAR T cells derived from healthy donors along with the traditional CAR T cell therapy using a patient’s own cells (autologous). Research developments like these can potentially bring down the cost of the treatment making it more accessible and affordable. As research and development continues, there’s hope that CAR T-cell therapy will become the most recommended treatment option, improving outcomes at large.

 oncologist. He has worked in the departments of Medical Oncology including Pediatric Oncology and Stem Cell Transplant of both Tata Memorial Centre and AIIMS. He is recognized for administering treatment from first clinical assessment of the patient to planning appropriate investigations, deciding the treatment, monitoring of chemotherapy, management of toxicities, response evaluations and follow-ups.
oncologist. He has worked in the departments of Medical Oncology including Pediatric Oncology and Stem Cell Transplant of both Tata Memorial Centre and AIIMS. He is recognized for administering treatment from first clinical assessment of the patient to planning appropriate investigations, deciding the treatment, monitoring of chemotherapy, management of toxicities, response evaluations and follow-ups.