Physics — the crux to finding new cancer therapies
A purview of current solutions and future scope
By Dr. K R Muralidhar, Director of Physics, Karkinos Healthcare
With the existing treatment modalities, there has not been a significant impact on cancer treatment or survival. Worldwide, an estimated 19.3 million new cancer cases and almost 10 million cancer deaths occurred in 2020. Female breast cancer has surpassed lung cancer as the most commonly diagnosed cancer, with an estimated 2.3 million new cases. This clearly shows the failure or inefficiency of the existing modality, either in diagnosis or in treatment, for most of the cancer treatments. So, why is cancer treatment this difficult and in this context what role and scope can physics play in the evolution of new treatment options for cancer?
To answer these questions, we need to first understand the biology of cancer cells. The foundation of modern cancer biology rests on a simple principle –– cancer is a result of uncontrolled proliferation of cells in certain tissues of the human body. All the cells in the human body have the same genome (DNA), which all have the same genetic wiring. So, if we understand how normal cells behave and what components of the cell regulate this behaviour through the genome (i.e., DNA), we can understand what goes wrong in cancer and then design appropriate methods of treatment that can target these components to destroy cancer.
Hence, there are 3 objectives in our efforts to improve the number of treated cancer cases.
They are:
- To analyse the failures in current modality of cancer diagnosis and treatment and its drawbacks.
- Estimating physics’ role and possible solutions in future cancer therapy modalities.
- Role of newer technologies in treating cancer.
The need for newer treatment modalities
In the present treatment modalities of cancer, we have been using century-old basics like surgery, radiotherapy (gamma rays, x-rays, and ion therapy) and chemotherapy. Of course, there has been substantial improvement in care done by surgery, radiotherapy, and chemotherapy for the past few years.
In the year 1895, W. C. Roentgen discovered X-rays and within months, systems were being devised to use x-rays for diagnosis. In the next 3 years, radiation was used to treat cancer. In the meantime, between 1896 and 1898, radioactive substances like ‘polonium’ and ‘radium’ were discovered. Sadly, till date, there exists no other treatment procedure and diagnosis apart from these basic modalities.
The burning question is despite using these time-tested modalities, why have we not been able to completely treat cancer? This is because the tumour response is different in each individual due to several factors including hypoxia, autopsy, angiogenesis, and cell proliferation. The periphery of the tumour at cellular level is not confirmed by any technology including, CT, MRI, and PET-CT scans that are routinely used for diagnosis. Also, the existing detectors cannot detect the changes at the level of cell and DNA. Unlike some other pathologies, cancer is a term that groups many different diseases into a single classification. The molecular mechanism is different for everyone depending on the genetic background, tissue type, tumour stage, even amongst cancers of the same type. The high mutation rates and genomic instability associated with many cancer cells allow them to adapt to the presence of treatment. These factors, therefore, make the cancer treatment unsuccessful in most of the cases. Hence, the future treatment modalities can include:
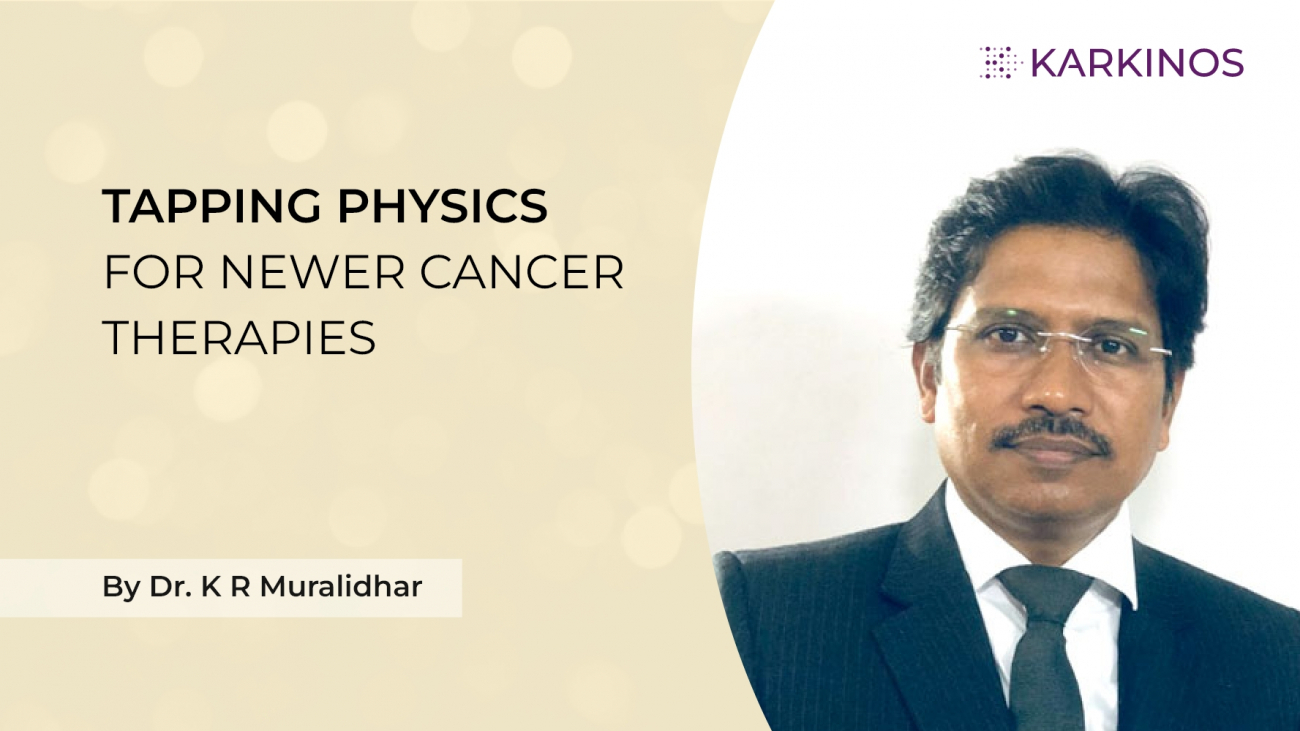
1. Personalized medicine: All cancer patients do not have the same prognosis. So, each patient requires a personalized treatment plan, and not the same treatment as others. The patient’s tumour type will be profiled and a corresponding personalized therapy regime will be prescribed according to their own specific cancer type. Eg: Cancer genomics has the potential to make personalized therapy a reality.
2. Contact inhibition: Contact inhibition is a property that helps normal, noncancerous cells to cease proliferation and growth when they come in contact with each other. This characteristic is lost when cells undergo mutations, leading to uncontrolled proliferation and solid tumor formation. Tumor cells then begin migrating to other parts of the body. The physical interactions between a cell and the extracellular matrix have a key role in allowing cells to migrate from a tumour to nearby blood vessels.
During intravasation and extravasation, cells must undergo large elastic deformations to penetrate endothelial cell-cell junctions. In the vascular system, the interplay between cell velocity and adhesion influences the binding of cancer cells to blood vessel walls and hence the location of sites where a secondary tumour can form and grow. An understanding of the role of physical interactions and mechanical forces, and their interplay with biochemical changes will provide new and important insights into the progression of cancer and may provide the basis for new therapeutic approaches.
Also, the role of physics like mechanical forces, extracellular matrix (ECM) stiffness and the ECM pore size and tortuosity play a major role in cell response to drugs. Several drug candidates show potential when examined in vitro but fail in clinical trials. This failure may stem at least in part from the use of conventional in vitro systems that fail to replicate the physiological microenvironment in humans as well as the lack of cell-phenotypic measurements. Here physics comes into picture to explore the cell response to the drug in a systematic fashion.
3. Medicinal chemistry: Medicinal chemistry combines chemistry, biology, and pharmaceutical sciences to design, develop, and examine new treatments for various diseases. Usually, a drug has to be absorbed to get into the blood circulation and to diffuse to various tissues and organs in the body, without undergoing substantial biochemical modification or degradation (metabolism), to finally bind to a specific macromolecule (target protein) and exert the desired therapeutic action.
The average mammalian cell volume is 𝟒 𝐱 𝟏𝟎−𝟗 cm3. One human cell contains 𝑨 = 𝟏.𝟑 𝐱 𝟏𝟎𝟏𝟒 atoms. Even with today’s most powerful computers, a classic in vitro simulation of a physical model for a cell that contains this many atoms is practically impossible. The situation becomes worse when considering the many different molecular components besides water that exist in real cells. Physics, therefore is critical to:
Identify and quantify the molecular interactions between small molecules (drugs) and biological targets (receptors) in situ;
Knowing thermodynamics of drug receptor interactions and so on;
Estimating the right intermolecular properties at macromolecular level; and
Deriving the principles and molecular mechanism underlying the therapeutic action of drugs.
4. Therapeutic antibodies: Antibodies are large proteins that are made by our immune system to help fight foreign invaders. They bind tightly to a particular region on the foreign substance (antigen) at a corresponding antigen receptor protein area. Developing these antibodies is one possibility to fight cancer through one of the physics applications. Eg: Photo Immunotherapy.
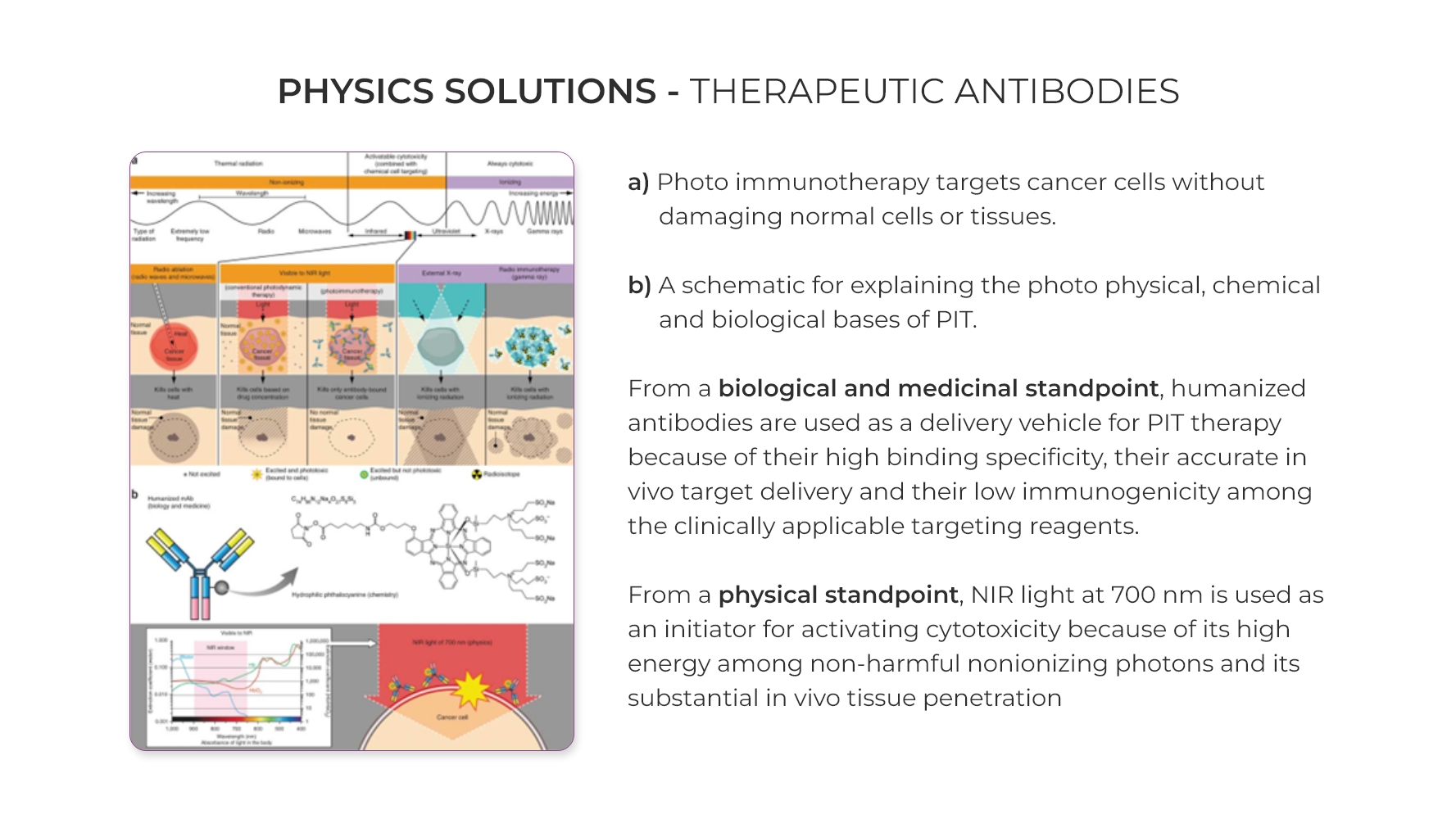
Photo immunotherapy targets cancer cells without damaging normal cells or tissues. From Physics standpoint, Near-Infrared (NIR) light at 700 nm is used as an initiator for activating cytotoxicity because of its high energy among non-harmful non ionizing photons and its substantial in vivo tissue penetration.
5. Epigenetics: The basic principle of epigenetics is that not all genes are active or ‘expressed’ at the same time. Modifications to the DNA that do not involve changing the actual sequence of adenine, cytosine, guanine and thymine influence the switching on and off genes.
To achieve stability and heritability of epigenetic states, cells take advantage of many different physical principles (dynamical system and the electrostatic and mechanical properties) related to chemical modifications of DNA and histones. By putting the complex biological literature from physics’ perspective by shaping, and transmitting not only allows one to rationalize the normal cellular functions, but also helps to understand the emergence of pathological states.
6. New horizons in diagnosis using mass spectrometry: Breath test is a recent development that uses mass spectrometry to test for compounds produced by lung cancer cells. This could, in future, allow doctors to diagnose lung cancer much earlier, and in a non-invasive, pain-free way. The device works by measuring volatile organic compounds (VOCs) at low concentrations in a patient’s breath and offers a cheaper and smaller alternative to existing detection technologies.
7. Endo photonics: Photonics is the science of light (photon) generation, detection, and manipulation through emission, transmission, modulation, signal processing, switching, amplification, and detection/sensing.
Though covering all light’s technical applications over the whole spectrum, most photonic applications are in the range of visible and near-infrared light. Endo photonics system is built on the Raman Spectroscopy technique – a vibrational technique capable of probing molecular changes associated with cellular transformation when tissue molecules were ‘excited’ by laser light directed at the tissue. The Raman technique has shown great promise for histopathologic assessments (i.e, optical biopsy) at the molecular level. Endo photonics is currently the trailblazer with the world’s first Raman Spectroscopy endoscopic device capable of capturing molecular signatures of tissues during endoscopy. Spectral information acquired during the endoscopic examination is matched against a database to provide real time diagnosis almost instantaneously.
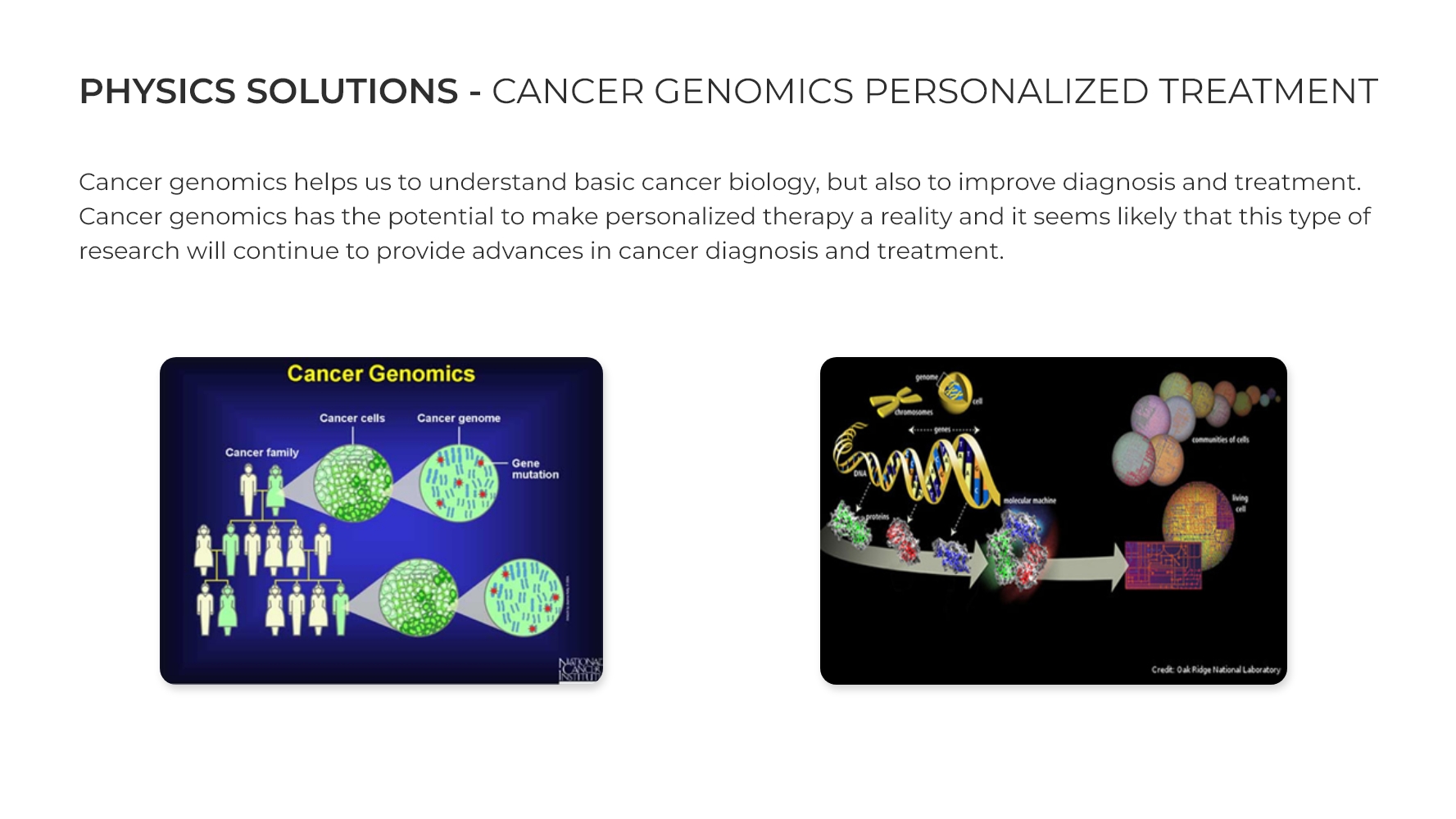
8. Cancer genomics: Cancer genomics helps us to understand basic cancer biology, but also to improve diagnosis and treatment. Cancer genomics has the potential to make personalized therapy a reality and it’s possible that this type of research will continue to provide advances in cancer diagnosis and treatment. A physics and biology driven and mathematics based design of the engineered drug delivery multiplies the probability of recognition of the novel targets that provides a synergistic solution for the imaging and therapy of the tumours at the interface of physics, cancer biology, engineering and mathematics.
9. Bioinformatics: Bioinformaticians design and implement computer programmes or data analysis algorithms that can be used to extract biologically meaningful relations from the large amounts of data generated by more than high-throughput measurement techniques used in genomics and transcriptomics.
As the field expands, and focus shifts to inferring 3D structures based on primary sequences and data obtained from other types of structural experiments, tremendous opportunities for young scientists with various expertise in the physical sciences.
Opportunities in three separate but overlapping fields:
1. comparative genomics (sequence analysis),
2. protein modeling (x-ray crystallography and NMR spectroscopy),
3. computational proteomics (protein folding, conformational sampling, docking, and molecular dynamics simulations).
These areas are considered to be the hot fields that can generate employment opportunities for bio-mathematicians and statisticians, computer scientists, chemists, computational biochemists, and physicists. Right now, the focus is on comparative genomics. But as the research focus begins to shift, new opportunities are expected to surface.
10. Molecular genetics: Cell signalling, stimulation of blood vessels, metabolism and immune systems are some of the key hallmarks of cancer study. Cancers are complex that deregulate some of the main molecular genetic pathways that control normal cell behaviour. If detailed studies on how these pathways work are done, we can then design drugs that are specifically targeted at these mutations in cancer cells.
Recent advances in our ability to watch the molecular and cellular processes of life in action — such as atomic force microscopy, optical tweezers and Forster Fluorescence Resonance Energy Transfer — raise challenges for Digital Signal Processing (DSP) of the resulting experimental data. It exposes the problems with classical linear DSP algorithms applied to this kind of data, and describes new nonlinear and non-Gaussian algorithms that are able to extract information that is of direct relevance to biological physicists. It is argued that these new methods applied in this context typify the nascent field of biophysical DSP.
11. Molecular mechanism: The molecular mechanism is different for each individual depending on genetic background, tissue type, tumour stage, even amongst cancers of the same type. Almost all processes in a living cell are performed by molecular machines. Eg: Actomyosin is the molecular motor that makes muscles move. The solution from the physics is that Actomyosin can be modelled using the formalism of diffusion and chemical reactions. A mathematical model describes the actomyosin operating cycle as a sequence of states and the transitions between them.
12. Imaging modalities using nanotechnology: In vivo carbon nanotube sensors, which can either be intravenously injected into the bloodstream or implanted under the skin, could be used to detect molecules like nitric oxide in the body – in real time. Fluorescent nanoparticles for quantitative uptake studies and Raman scattering gives bio imaging a boost.
13. Treatment modalities using nanotechnology: Recent studies show the advantage of using nanotechnology to treat cancer. As an example, some of the nanoparticles slow down cancer cells and gold nano-matryoshkas kill cancer cells.
14. Machine Learning and artificial intelligence in radiation oncology: We have achieved tremendous technical growth for fine delivery of Megavoltage therapy, electron therapy, Brachytherapy, Intra operative therapy. Technologies like IGRT, IMRT, VMAT can spare normal tissues to some extent and can deliver the required drug dose to the target cells. But the main aim of cancer treatment is possible only with good knowledge and data on the biological effects of radiation for the combinations of radiation, technique, dose, patient, disease, and so on. This can improve personalized treatments. Right now various research institutes are collecting this data for better radiation oncology treatments by using Artificial Intelligence and Machine Learning. There is a lot of scope for research in this area for physics and biology.
15. Nuclear medicine and radiology: Nuclear medicine is a medical specialty that uses radioactive tracers to assess bodily functions and to diagnose and treat disease. These radioactive tracers are tracked by specially designed cameras. Single Photon Emission Computed Tomography or SPECT and Positron Emission Tomography or PET scans are the two most common imaging modalities in nuclear medicine. Research on using new radioactive isotopes and on newer and improved imaging technology aim to work towards predicting tumour cell proliferation status, better molecular imaging information that could guide better treatment decisions.
Radiology uses imaging technology for diagnosis. AI and Machine Learning provide quantitative tools that will increase the value of diagnostic imaging as a biomarker, increase image quality with decreased acquisition times, and improve workflow, communication, and patient safety.
Radiological Physics helps to understand medical imaging components, technology and parameters. Physics in combination with AI and Machine learning are trying to bring out optimal imaging results while ensuring the patient is safe from radiation.
Physics in the game of cancer care
As our understanding about cell biology expands, we must believe that medical physics’ day-to-day practice must reach at the DNA level, not just at the imaging level. For this to happen, a combination of cell biology and nanotechnology will play a vital role. Imaging, also, should be applied at the cellular level.
At the same time, traditional treatment modalities that are harmful to normal cells while treating cancer cells with unknown resultant dose to the target should be addressed. DNA and protein information need to be analysed to know the basic cause of cancer and for the best treatment modality. To enable all these and to see significant and meaningful progress in cancer treatment, physics will be playing an integral role.
The physical laws and principles that define the behaviour of matter are essential for developing an understanding of initiation and progression of cancer at all length scales. In reality our body is not constant so the problem. The physics aspects can reach a size of atom level. Even the microbes are in a size more than atoms. Hence there is every chance that we can reach microbe or protein level to control cancer in gene level. Therapeutics to the core of the tumor, as well as its distance metastasis, will all benefit from an application of physical science approaches to oncology; from mechanics to evolution, chemistry and nanotechnology. Thus successful integration of approaches from mathematics, physics, and engineering with cancer biology may be our best hope to understand complex systems of cancer and to develop effective strategies for treatment.